 Regulation of Raf activity by KRas dimerization dynamics
Dissects whether KRas dimerization is required for Raf activation using live-cell FRET.
Regulation of Raf activity by KRas dimerization dynamics
Dissects whether KRas dimerization is required for Raf activation using live-cell FRET.
Regulation of Raf activity by KRas dimerization dynamics
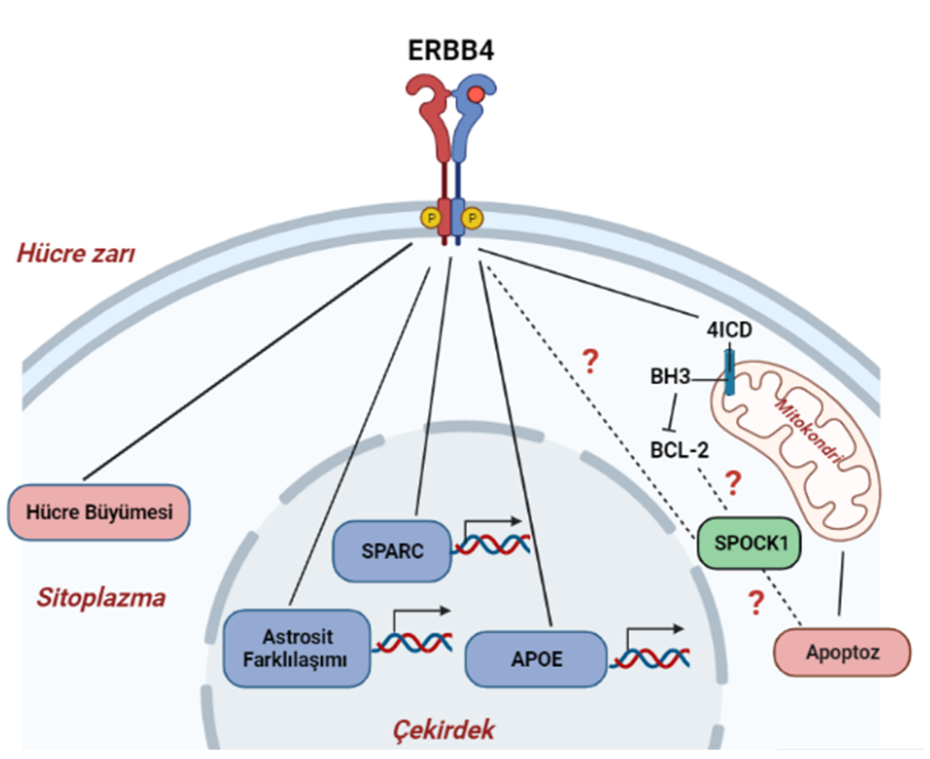
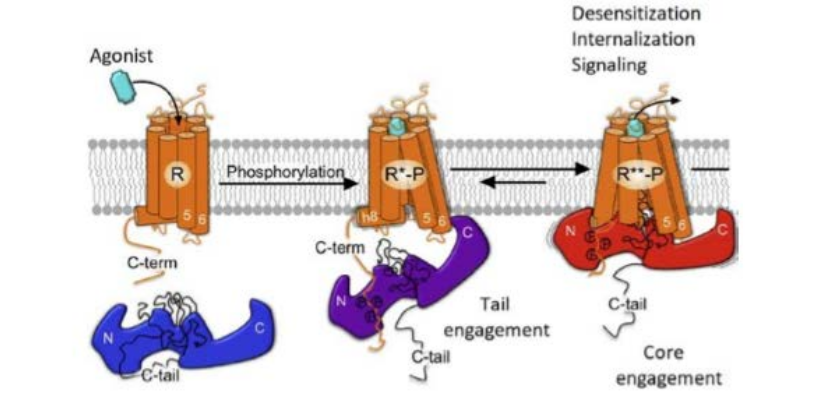
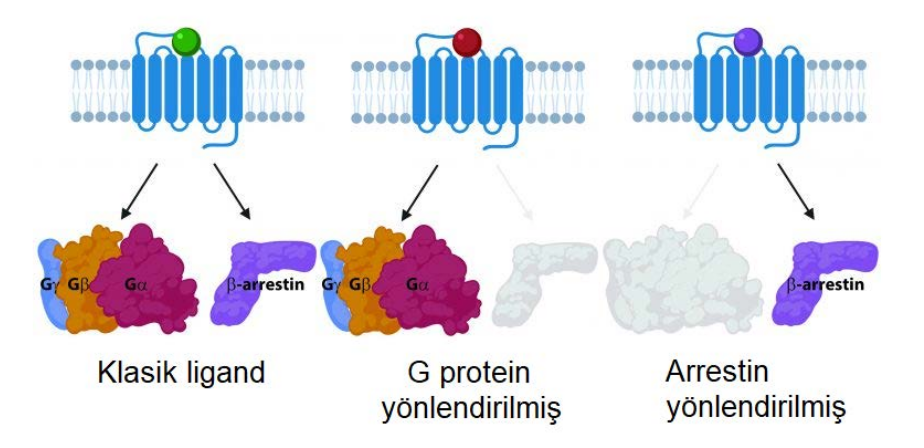
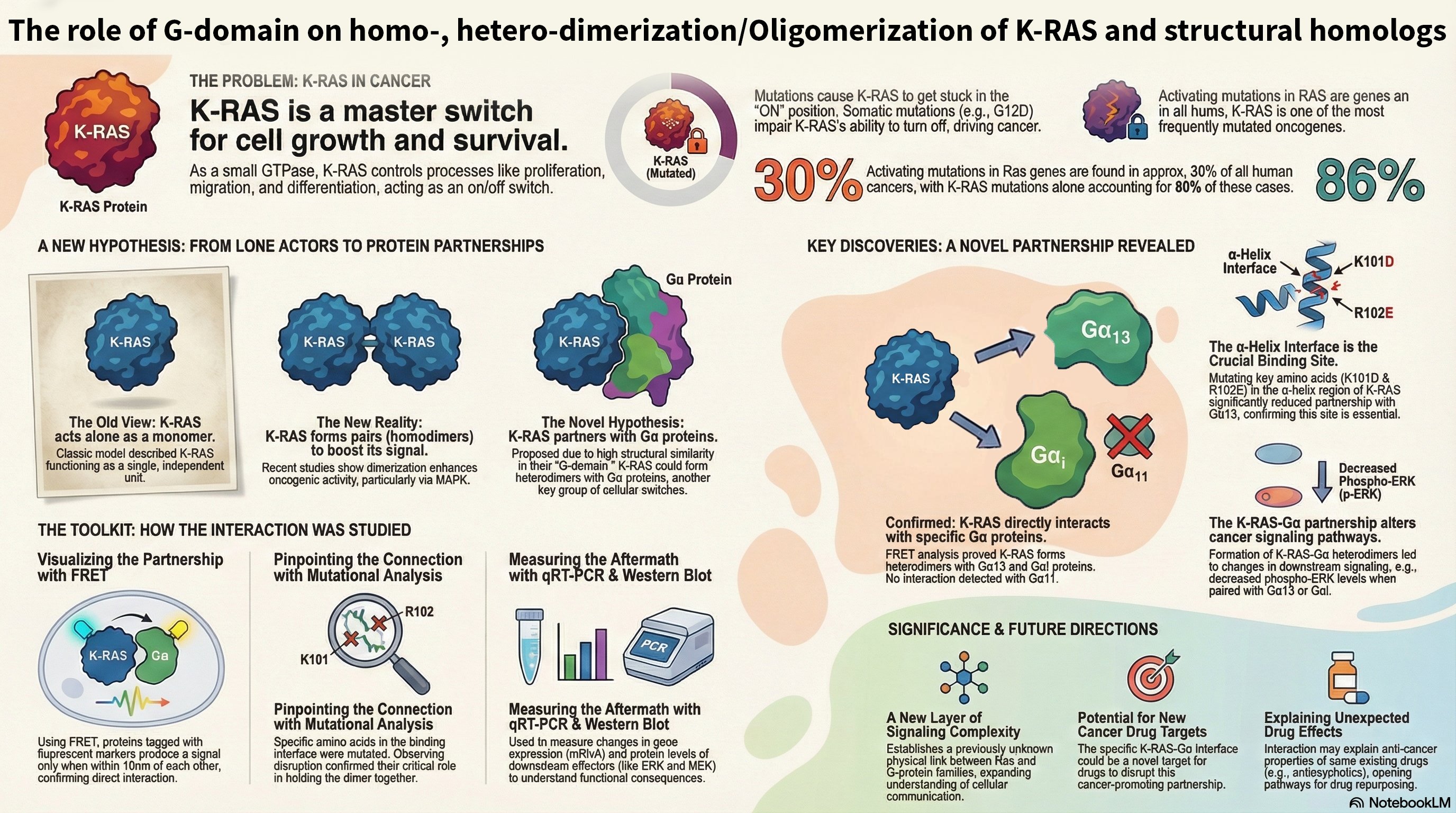
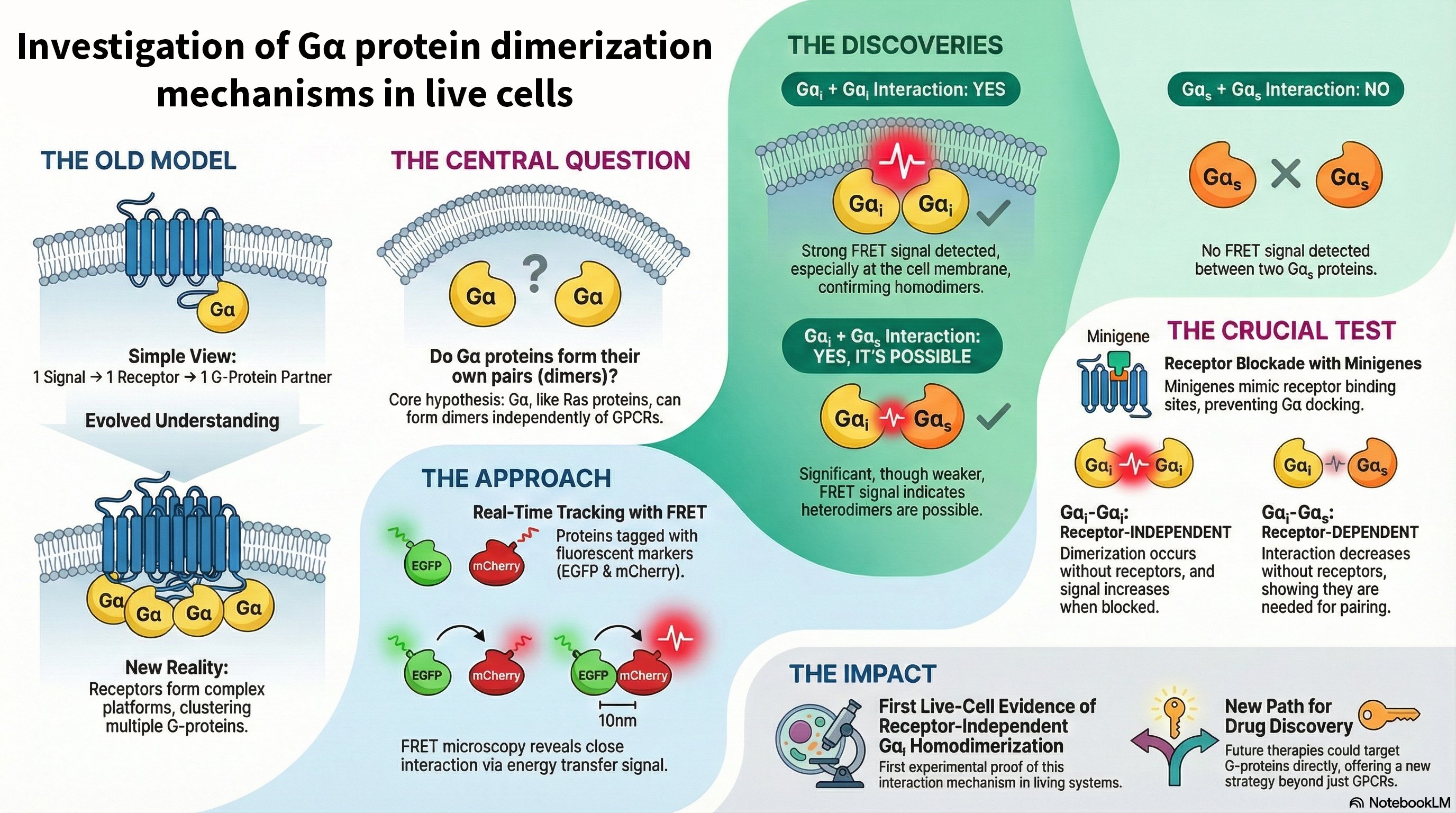
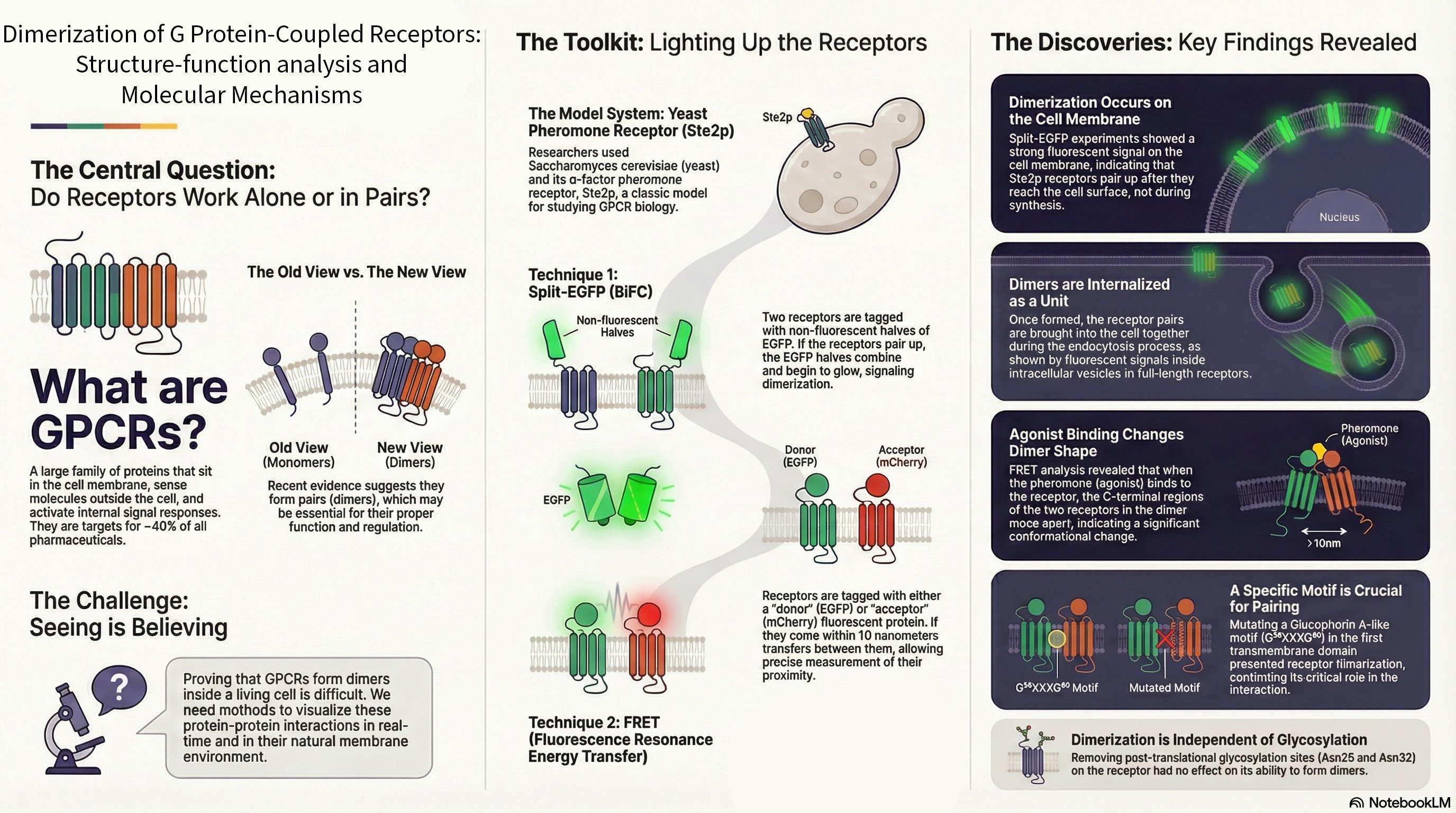
Overview: KRas is a critical GTPase in cellular signal transduction, regulating key pathways like MAPK/ERK and PI3K/AKT through its interaction with effectors such as Raf kinase. This project investigates the fundamental mechanism of Raf activation, specifically questioning whether KRas homodimerization is essential or if monomeric KRas is sufficient to drive downstream signaling. We explore the dynamics of KRas dimerization, potential compensatory mechanisms involving KRas-Gα heterodimerization, and how various KRas mutations influence signaling outcomes in both cancer and neuronal contexts.
Key Research Areas:
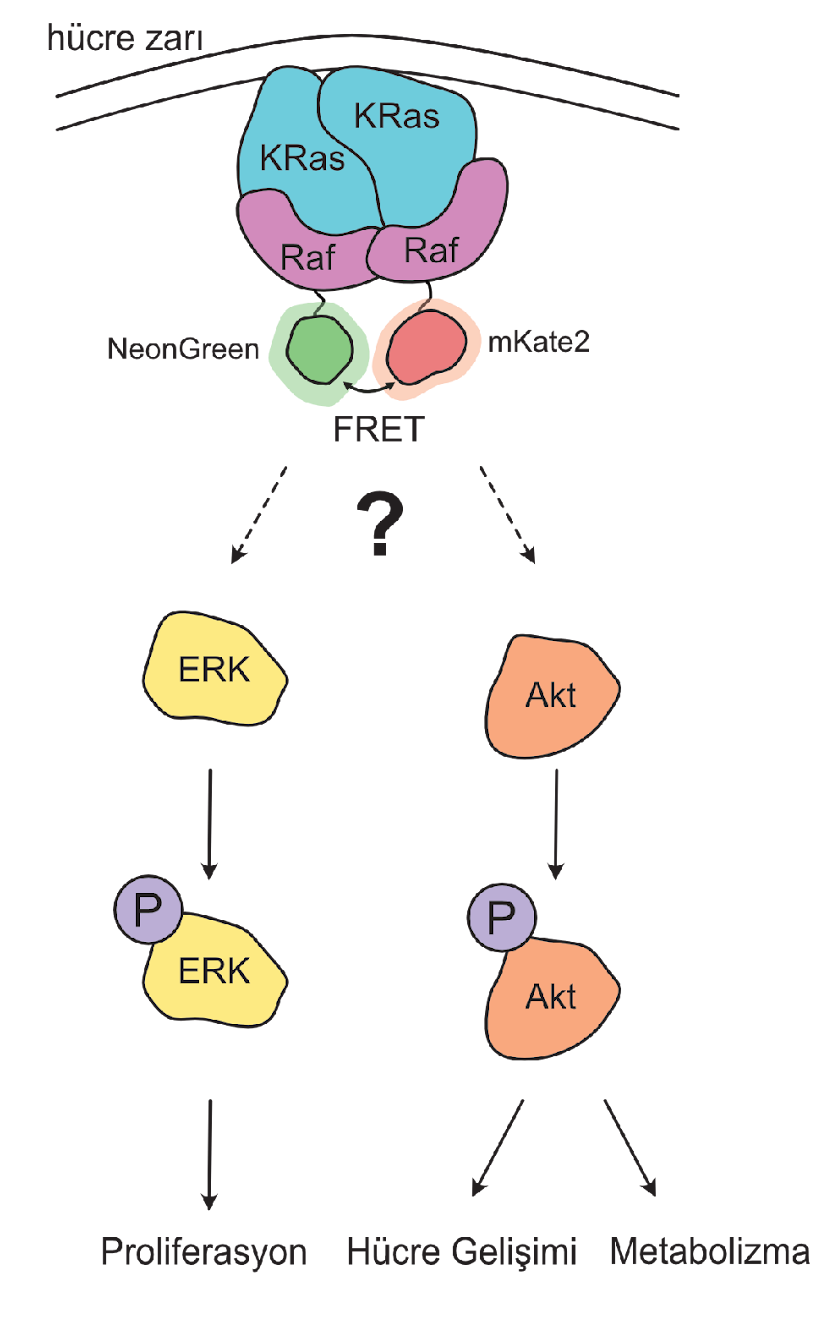
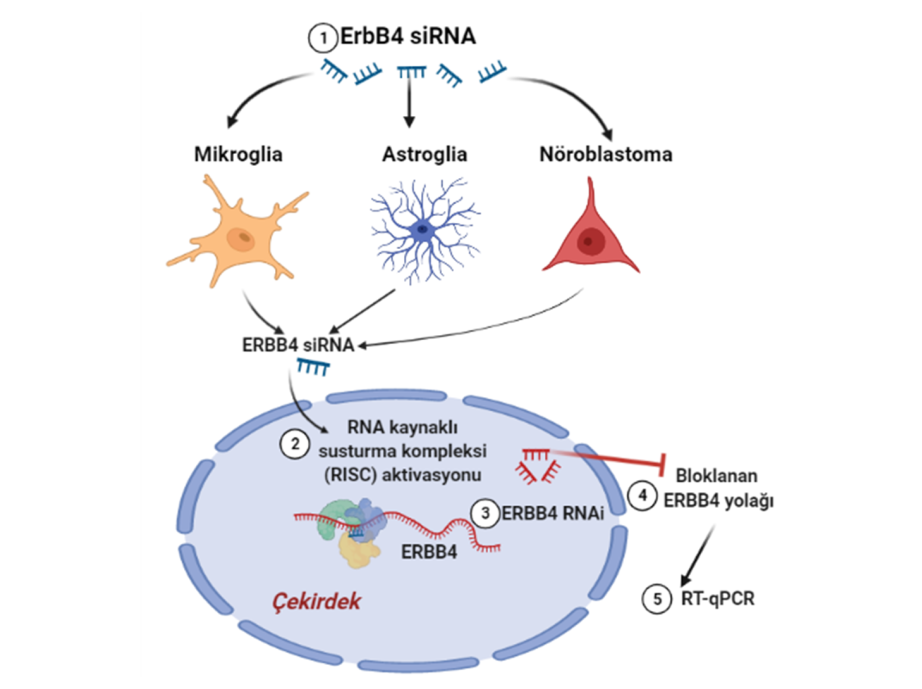
- Dimerization vs. Monomeric Activation: Using live-cell imaging and Förster resonance energy transfer (FRET), we are dissecting whether KRas monomers can activate Raf independently or if dimerization is required for a stable, prolonged signaling response.
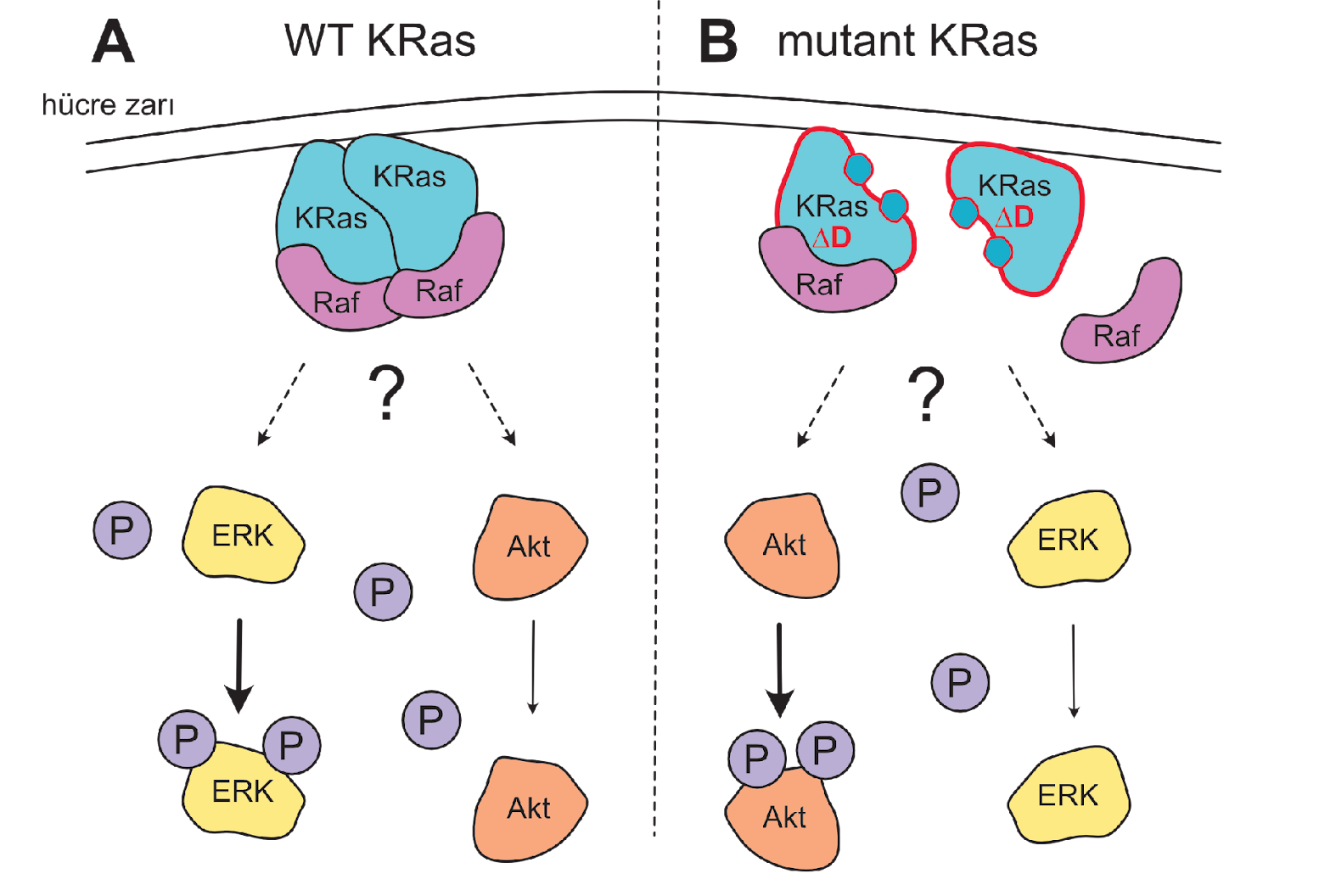
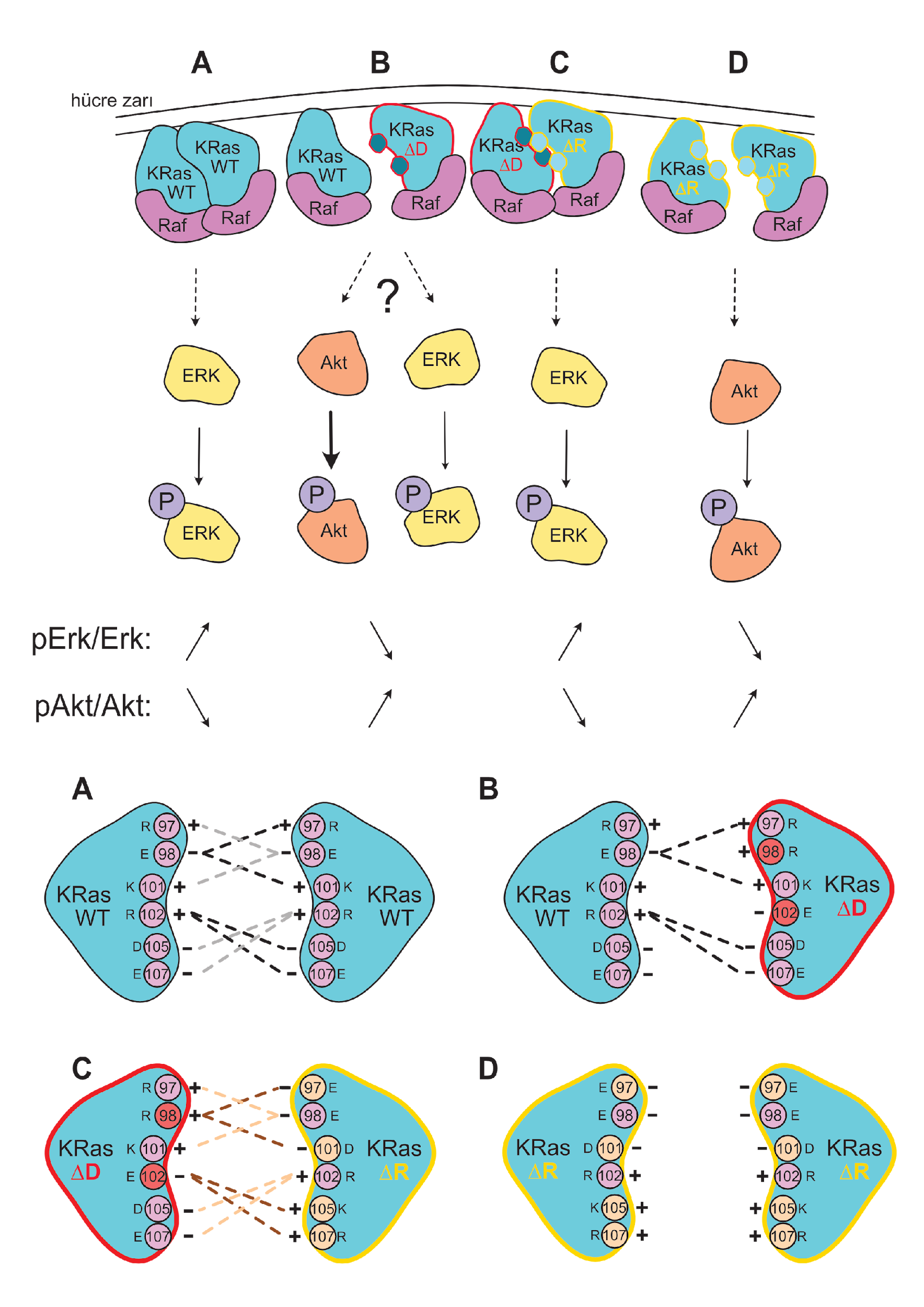
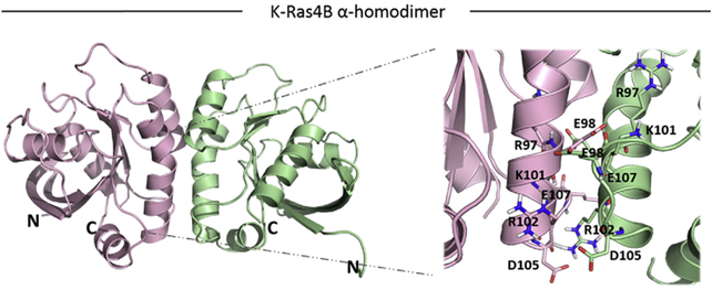
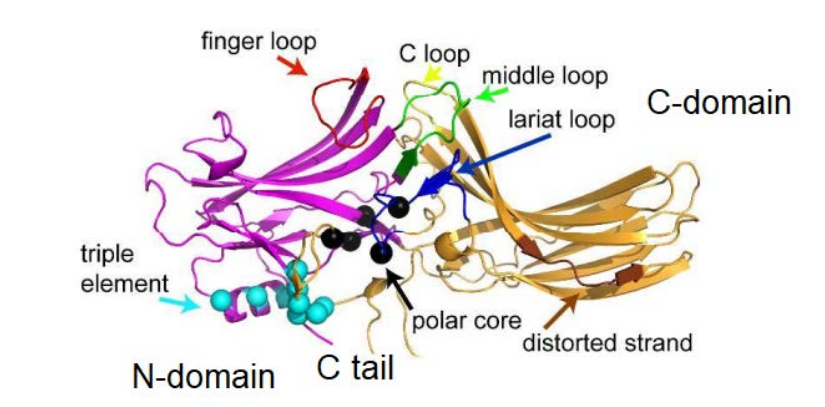
- Mutational Analysis of the Dimerization Interface: We employ precisely designed KRas mutations at key residues (e.g., Glu98, Arg97, Arg102) to selectively disrupt or rescue homodimerization. This genetic approach allows us to isolate the specific role of dimerization in signaling, a significant advantage over traditional chemical inhibitor methods.
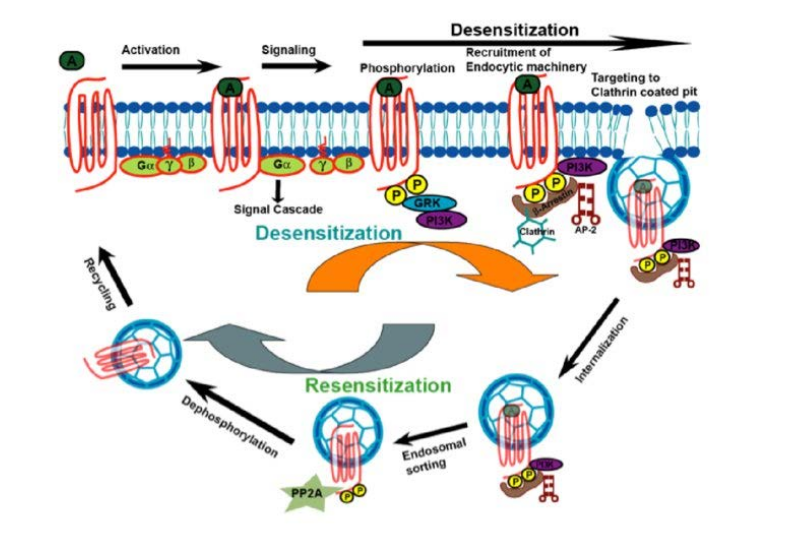
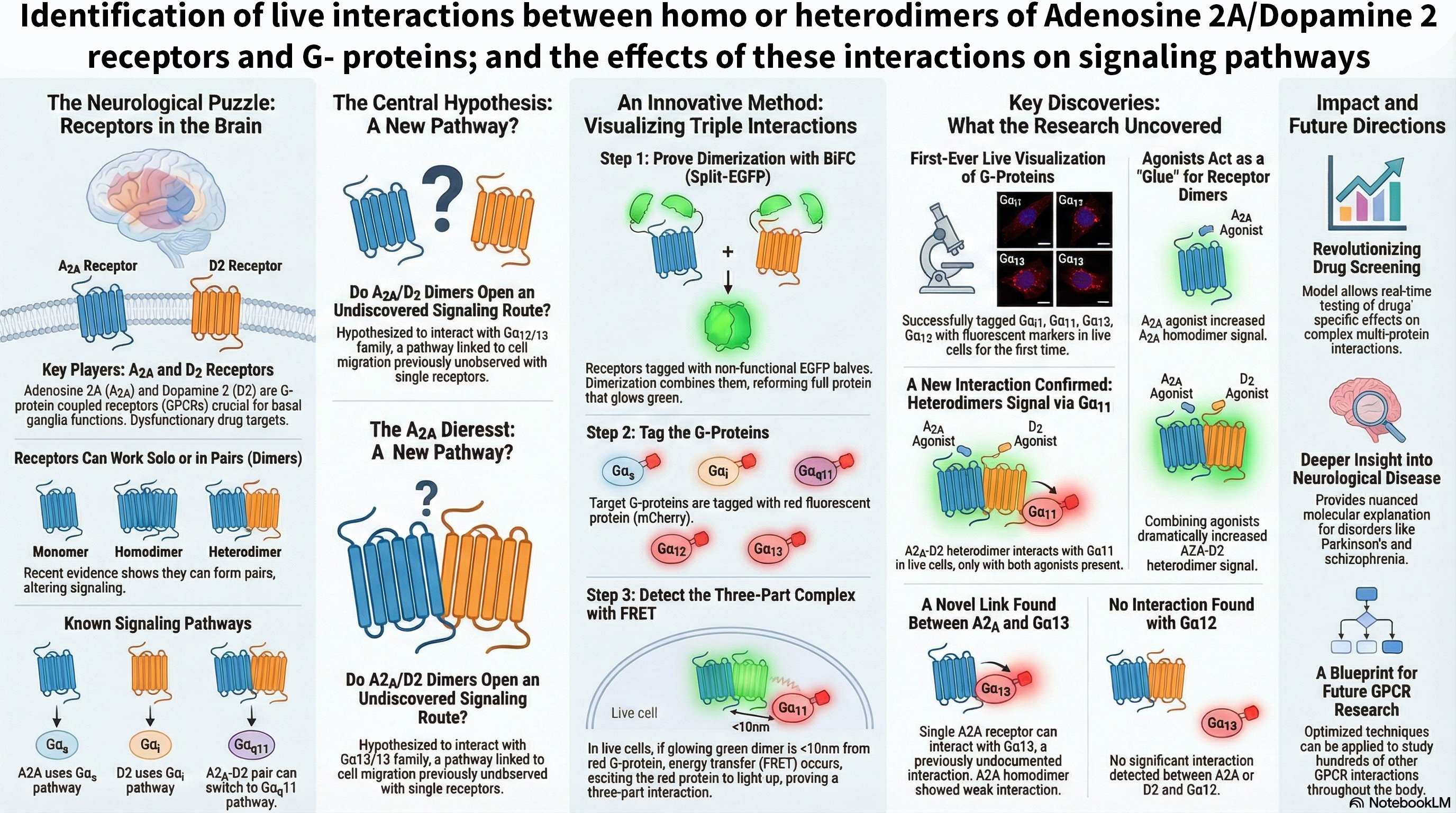
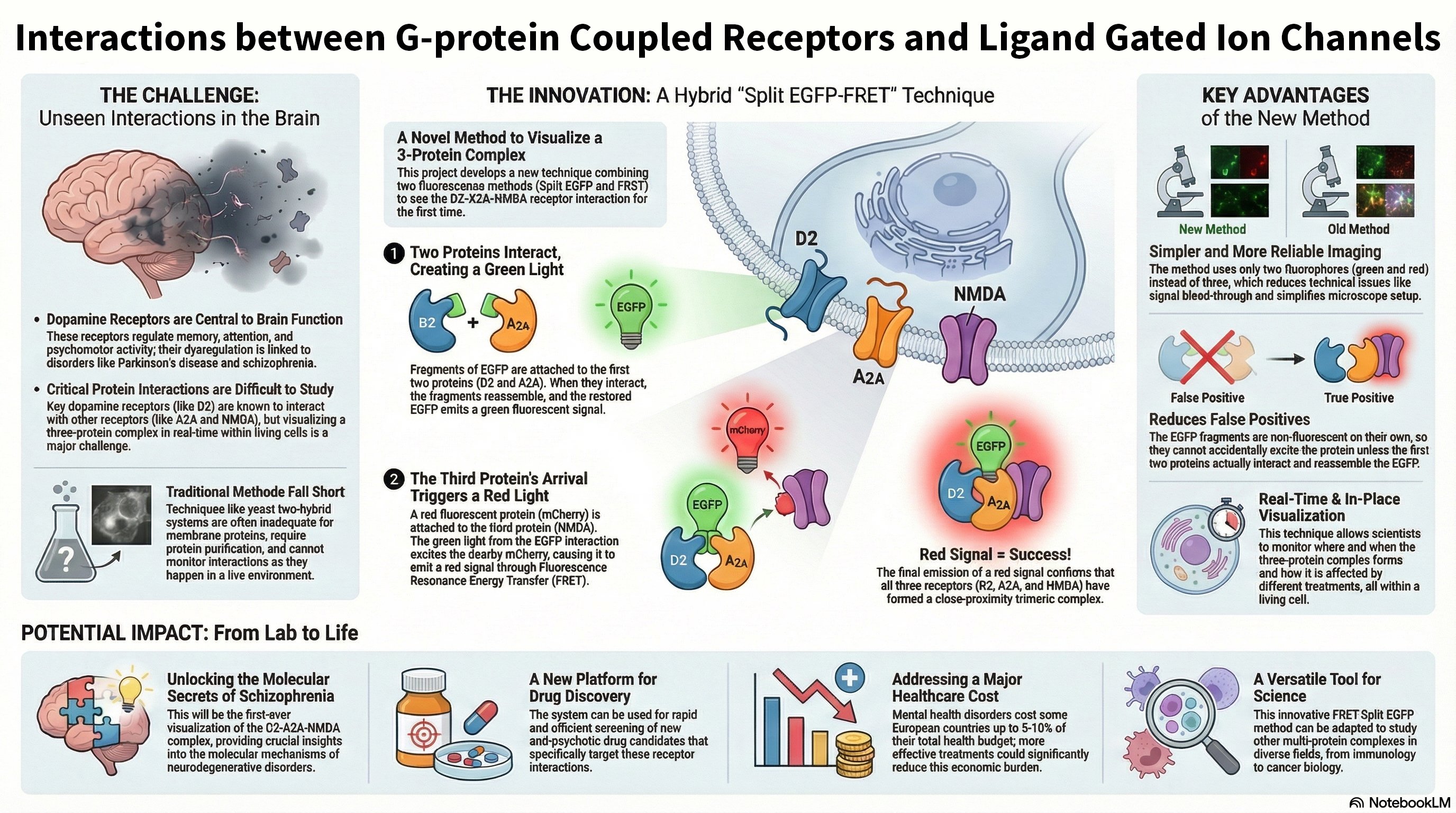
- Compensatory Mechanisms: We are investigating whether KRas-Gαi/Gα13 heterodimerization can serve as an alternative pathway to sustain Raf activation when homodimerization is compromised, potentially revealing novel cross-talk between GPCR and Ras signaling pathways.
Significance and Implications: By understanding the molecular nuances of KRas-driven signaling, this research aims to identify new regulatory nodes for therapeutic intervention in cancer, where KRas mutations are prevalent. Furthermore, given KRas's role in neuronal differentiation and synaptic plasticity, our findings could offer new insights into neurodevelopmental disorders and neurodegenerative diseases.

.jpg)